- About us
- A new Institute dedicated to combating leukemia
- Scientific and medical program
- History of Hematology on the Saint-Louis Campus
- The Leukemia Institute’s governance
- Press
- Contact us
- Our news
- Winners of the internal call for projects 2026
- Perspectives in the treatment of aplastic anemia
- The 2025 Olga Sain Prize
- Hugues de Thé’s Wishes
- Profile of Vincent Bansaye, Professor at École Polytechnique
- Support us
- Join us
- You are
- Patients and relatives
- To receive care and support
- Become an expert patient
- Discover the Leukemia Institute
- Researchers
- Research
- Clinical trials
- Discover the Leukemia Institute
- Healthcare professionals
- Refer a patient
- Our clinical research
- Discover the Leukemia Institute
- Industry partners
- Discover the Leukemia Institute
- Translational research
- Donors
- Support us
- Discover the Leukemia Institute
- Care
- Patient care
- Being Treated at the Leukemia Institute
- Cancer treatments
- Supportive Care
- Open Multidisciplinary Meetings
- Our clinical services
- Saint-Louis Hospital – Department of adult hematology
- Saint-Louis Hospital – Hematology Transplant Unit
- Saint-Louis Hospital – Department of Pharmacology and Clinical Investigations
- Saint-Louis Hospital – Adolescent and Young Adult Unit
- Saint-Louis Hospital – Outpatient Hemato-oncogenetics Unit
- Saint-Louis Hospital – Department of senior hematology
- Robert-Debré Hospital – Department of pediatric hematology and immunology
- Necker Hospital – Department of Adult Hematology
- Cochin–Port Royal Hospital – Department of clinical hematology
- Avicenne Hospital – Department of clinical hematology and cell therapy
- Our medical laboratories
- Hematology Medical Laboratory, Michaela Fontenay
- Hematology Medical Laboratory, Jean Soulier
- Molecular Genetics Unit, Hélène Cavé
- Hematology Medical Laboratory, Vahid Asnafi
- Patient information
- Acute Myeloid Leukemias
- Acute Lymphoblastic Leukemias
- Myeloproliferative Neoplasms
- Myelodysplastic Syndrome
- Cancer treatments
- Supportive Care
- Psychological Support
- Research
- Our research teams
- Hugues de Thé’s team – Molecular pathology
- Raphaël Itzykson’s team – Functional precision medicine for leukemia
- Michaela Fontenay’s team – Normal and pathological hematopoiesis
- Françoise Pflumio’s team – Niche, Cancer, and Radiation in Hematopoiesis
- Sylvie Méléard’s team – Population Evolution and Interaction Particle Systems
- David Michonneau’s team – Translational Immunology in Immunotherapy and Hematology (TIGITH)
- Lina Benajiba’s team – Identification and targeting of extrinsic regulators of myeloid malignancies
- Karl Balabanian’s team – Lymphoid niches, Chemokines and Immuno-hematological syndromes
- Alexandre Puissant’s team – Molecular Mechanisms of Acute Myeloid Leukemia Development
- Stéphane Giraudier’s team – Chronic Myeloid Malignancies, Microenvironment & Translational Research
- Diana Passaro’s team – Leukemia & Niche Dynamics
- Camille Lobry’s team – Genetic and Epigenetic control of Normal and Malignant Hematopoiesis
- Jean Soulier’s team – Stem cell dysfunction and secondary AML
- Sylvie Chevret’s team – Biostatistics and clinical epidemiology
- Our technological platforms
- Our clinical research
Accueil Care for patients with leukemia and related disorders Our clinical research Participate in a clinical trialParticipate in a clinical trial
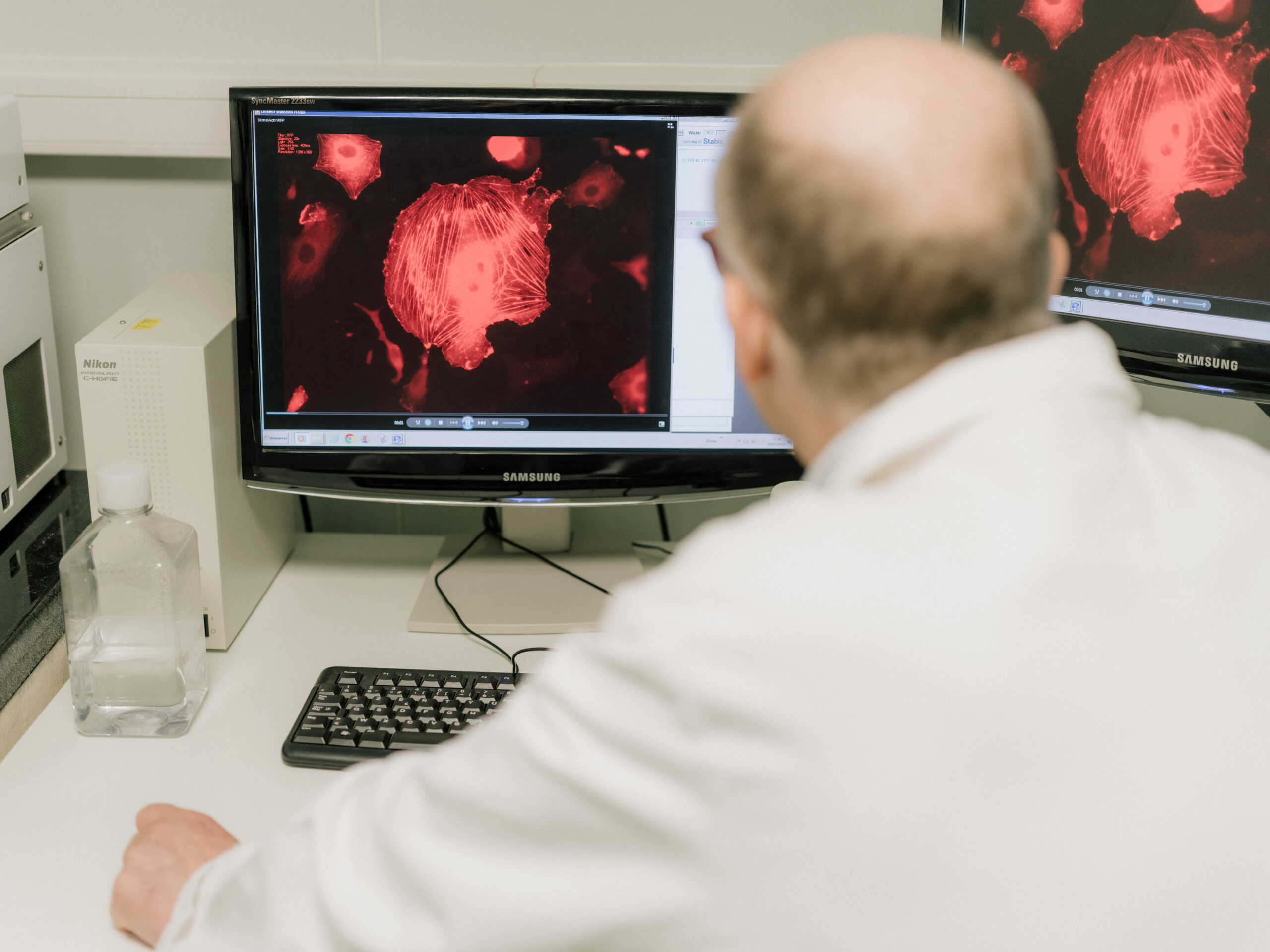
...Clinical research aims to improve medical knowledge and healthcare. It allows new treatments, medical devices, diagnostic methods, and care approaches to be evaluated.
A clinical trial is a study conducted with volunteers (patients or healthy individuals) to test a treatment or medical intervention. It follows a specific protocol and is strictly regulated by law.
Clinical trials
Your doctor or healthcare team may suggest that you participate because you
meet the necessary criteria for this study (age, type of leukemia, medical history, etc.). This does not mean that your current treatment is insufficient, but that another option could be tested in a safe environment.You will receive a document detailing all the necessary information:
- the objectives of the study,
- its duration,
- potential benefits and risks,
- and requirements (exams, visits,
questionnaires, etc.) during a consultation.
This document is often long and contains terms that can be complicated. Reading it may cause you to feel anxious. Take the time to read the information, write down your questions, and ask the team for explanations.
If you wish to participate in the research protocol, you will sign an informed consent form after you have had the opportunity to ask your questions.
Certain requirements may exist and vary depending on the study.
For example:
- Not knowing which treatment you will receive after randomization.
- A greater number of consultations or hospital visits.
- Additional medical examinations, which may sometimes be redundant.
- Regular medication or questionnaires to complete.
- Uncertainty about the effectiveness of the proposed treatment.
All this information will be clearly explained to you before you make your decision. You will be able to ask the research team any questions you may have.
No.
Participation is entirely voluntary. You can withdraw from the study at any time during the protocol. You can also refuse to participate without this affecting the quality of your care.
You could benefit from innovative treatment, receive enhanced medical monitoring, and contribute to improving care for other patients.
As with any treatment, the drugs or techniques being tested may have side effects.
The study is supervised to minimize risks and monitor them continuously.Yes. You are free to discontinue your participation at any time, without justification and without any impact on your medical care.
Protection, controls, and funding of clinical trials
Personal and medical data are strictly confidential.
They are protected in accordance with current regulations
(GDPR).The research team remains available throughout the study to answer any questions.
It is composed of clinical research assistants (CRAs). They are not healthcare professionals but manage the entire study and can provide guidance and answer questions.
If specific care is required for the protocol, it is provided by clinical research nurses who are specifically trained for the protocol.
Trials are authorized by independent ethics committees and monitored by health authorities. They are conducted by trained and experienced teams.
Clinical trials can be funded by:
- Public organizations (hospitals, research institutes, universities, etc.)
- Patient associations or foundations
- Pharmaceutical or healthcare companies
Regardless of the funder, all studies must comply with the same ethical, scientific, and regulatory rules.
Glossary
An independent group composed of physicians, researchers, patients, and citizens, responsible for verifying that the study respects the rights and safety of participants.
Document signed by the participant to confirm that they have understood the study and that they agree to participate voluntarily.
Requirements for participating in a study (age, illness, medical history, etc.).
Medical or administrative information about you, protected by law and used solely for research purposes.
A study that evaluates a treatment, medication, technique, or healthcare organization.
A substance with no therapeutic effect used as a comparison in certain studies to evaluate the actual effectiveness of a treatment.
A document that describes in detail the objectives, conditions for implementation, duration, methods, and evaluation criteria of the study. This term is sometimes used to refer to the research itself.
Random assignment of participants to different treatment groups to ensure impartiality of results.
Stay up to date by subscribing to the institute's newsletter
- Discover the Leukemia Institute
- Translational research
- Our clinical research
- Clinical trials
- Become an expert patient
- To receive care and support
- A new Institute dedicated to combating leukemia