- About us
- A new Institute dedicated to combating leukemia
- Scientific and medical program
- History of Hematology on the Saint-Louis Campus
- The Leukemia Institute’s governance
- Press
- Contact us
- Our news
- Profile of Valéria Bisio, Research Officer
- Creation of a Hemato-Oncogenetics Unit
- Profile of Alice Gros, a peer-support patient
- Winners of the Leukemia Institute’s first call for projects
- Profile of Julien Calvo, researcher
- Support us
- Join us
- You are
- Patients and relatives
- To receive care and support
- Become an expert patient
- Discover the Leukemia Institute
- Researchers
- Research
- Clinical trials
- Discover the Leukemia Institute
- Healthcare professionals
- Refer a patient
- Our clinical research
- Discover the Leukemia Institute
- Industry partners
- Discover the Leukemia Institute
- Translational research
- Donors
- Support us
- Discover the Leukemia Institute
- Care
- Patient care
- Being Treated at the Leukemia Institute
- Cancer treatments
- Supportive Care
- Open Multidisciplinary Meetings
- Our clinical services
- Saint-Louis Hospital – Department of adult hematology
- Saint-Louis Hospital – Hematology Transplant Unit
- Saint-Louis Hospital – Department of Pharmacology and Clinical Investigations
- Saint-Louis Hospital – Adolescent and Young Adult Unit
- Saint-Louis Hospital – Outpatient Hemato-oncogenetics Unit
- Saint-Louis Hospital – Department of senior hematology
- Robert-Debré Hospital – Department of pediatric hematology and immunology
- Necker Hospital – Department of Adult Hematology
- Cochin–Port Royal Hospital – Department of clinical hematology
- Avicenne Hospital – Department of clinical hematology and cell therapy
- Our medical laboratories
- Hematology Medical Laboratory, Michaela Fontenay
- Hematology Medical Laboratory, Jean Soulier
- Molecular Genetics Unit, Hélène Cavé
- Hematology Medical Laboratory, Vahid Asnafi
- Patient information
- Acute Myeloid Leukemias
- Acute Lymphoblastic Leukemias
- Myeloproliferative Neoplasms
- Myelodysplastic Syndrome
- Cancer treatments
- Supportive Care
- Psychological Support
- Research
- Our research teams
- Hugues de Thé’s team – Molecular pathology
- Raphaël Itzykson’s team – Functional precision medicine for leukemia
- Michaela Fontenay’s team – Normal and pathological hematopoiesis
- Françoise Pflumio’s team – Niche, Cancer, and Radiation in Hematopoiesis
- Sylvie Méléard’s team – Population Evolution and Interaction Particle Systems
- David Michonneau’s team – Translational Immunology in Immunotherapy and Hematology (TIGITH)
- Lina Benajiba’s team – Identification and targeting of extrinsic regulators of myeloid malignancies
- Karl Balabanian’s team – Lymphoid niches, Chemokines and Immuno-hematological syndromes
- Alexandre Puissant’s team – Molecular Mechanisms of Acute Myeloid Leukemia Development
- Stéphane Giraudier’s team – Chronic Myeloid Malignancies, Microenvironment & Translational Research
- Diana Passaro’s team – Leukemia & Niche Dynamics
- Camille Lobry’s team – Genetic and Epigenetic control of Normal and Malignant Hematopoiesis
- Jean Soulier’s team – Stem cell dysfunction and secondary AML
- Sylvie Chevret’s team – Biostatistics and clinical epidemiology
- Our technological platforms
- Our clinical research
Accueil First leukemia research center in France Hugues de Thé’s team – Molecular pathologyHugues de Thé’s team – Molecular pathology
...Hugues de Thé
Team leader
Institut de recherche Saint-Louis

Research themes
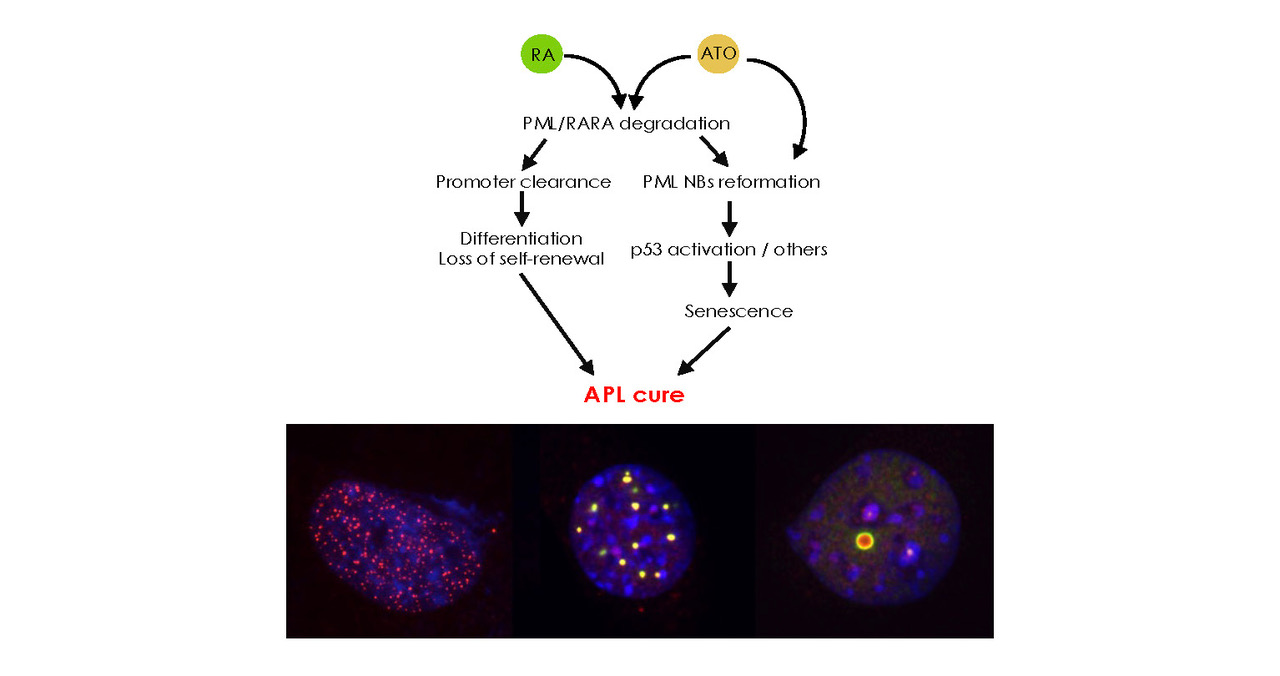
Our team aims to decipher the cellular and molecular mechanisms of therapeutic response in acute myeloid leukemia. Our model of choice is the response of acute promyelocytic leukemia to retinoic acid and arsenic. Recent work focuses on the response to conventional chemotherapy.
Research areas
We have demonstrated that Pml controls sumoylation, coupling it to oxidative stress. Furthermore, normal Pml protein is essential for the PML/RARA fusion-induced LAP response. Work in progress shows that Pml is also important for the response to conventional chemotherapy, via the control of senescence. In both models, we are exploring the structure/function relationships of Pml (redox sensing site, sumoylation sites…).
In APL, efficient therapeutic response requires the presence of Pml, but disease initiation is linked to interference with RARA signaling. Indeed, diseases similar to classical PBL have been described in patients after overexpression of RARA following viral insertion. Previous studies suggest that transcriptional repression plays a role in immortalization. We aim to understand the molecular mechanisms of repression and the nature of the downstream targets involved in acquiring self-renewal and blocking differentiation.
We are pursuing two approaches in parallel. One uses mouse models of AML in which the Pml gene has been inactivated. These show a blocked response to conventional chemotherapy, associated with an absence of treatment-induced senescence. The other model explores the single-cell transcriptional response directly in patients. These converging approaches address the gene networks and signaling cascades mobilized in response to conventional chemotherapy.
Hugues de Thé's team members
Majdouline Abou-GhaliPost-doctoral felowPaolo AyakaPhD studentCaroline BerthierResearch engineer, Collège de FranceThassadite DiramiResearch scientist, UPCCécile EsnaultCRCN, INSERMOmar FerhiResearch engineer, INSERMMarie-Claude GeoffroyCRHC, CNRSWissem HamelContract engineerMichiko KawatikaResearch scientist, UPCHao LiangContract engineerAdèle MarmusePhD studentHassane SoilihiTechnician, CNRSHugues de ThéProfessor, Collège de FranceYi ZhangPost-doctoral fellowHsinchieh WuPost-doctoral fellowScientific publications
Pierre Bercier & al, Cancer Discover. 2023
Structural Basis of PML-RARA Oncoprotein Targeting by Arsenic Unravels a Cysteine Rheostat Controlling PML Body Assembly and FunctionRead the publicationHsin-Chieh Wu & al, Cancer Discover. 2021
Actinomycin D Targets NPM1c-Primed Mitochondria to Restore PML-Driven Senescence in AML TherapyRead the publicationAnthony Letai, Hugues de The, Nat Rev Cancer, 2025
Conventional chemotherapy: millions of cures, unresolved therapeutic indexRead the publicationStay up to date by subscribing to the institute's newsletter
- Discover the Leukemia Institute
- Translational research
- Our clinical research
- Clinical trials
- Become an expert patient
- To receive care and support
- A new Institute dedicated to combating leukemia