Azithromycin promotes relapse by disrupting immune and metabolic networks after allogeneic stem cell transplantation
Nicolas Vallet & al, Blood, 2022
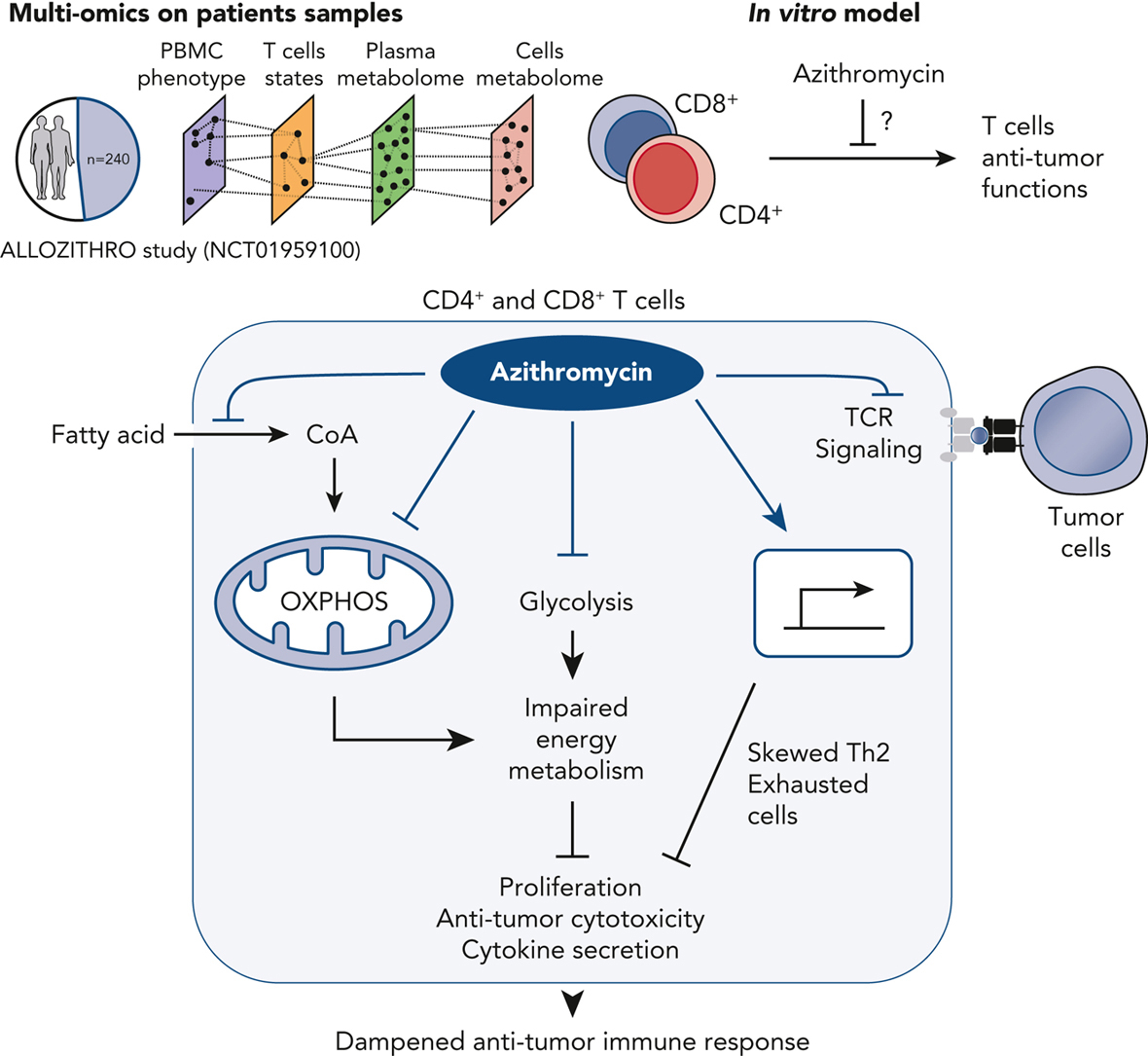
Administration of azithromycin after allogeneic hematopoietic stem cell transplantation for hematologic malignancies has been associated with relapse in a randomized phase 3 controlled clinical trial.
Studying 240 samples from patients randomized in this trial is a unique opportunity to better understand the mechanisms underlying relapse, the first cause of mortality after transplantation.
We used multi-omics on patients’ samples to decipher immune alterations associated with azithromycin intake and post-transplantation relapsed malignancies.
Azithromycin was associated with a network of altered energy metabolism pathways and immune subsets, including T cells biased toward immunomodulatory and exhausted profiles.
In vitro, azithromycin exposure inhibited T-cell cytotoxicity against tumor cells and impaired T-cell metabolism through glycolysis inhibition, down-regulation of mitochondrial genes, and up-regulation of immunomodulatory genes, notably SOCS1.
These results highlight that azithromycin directly affects immune cells that favor relapse, which raises caution about long-term use of azithromycin treatment in patients at high risk of malignancies.
The ALLOZITHRO trial was registered at www.clinicaltrials.gov as #NCT01959100.